
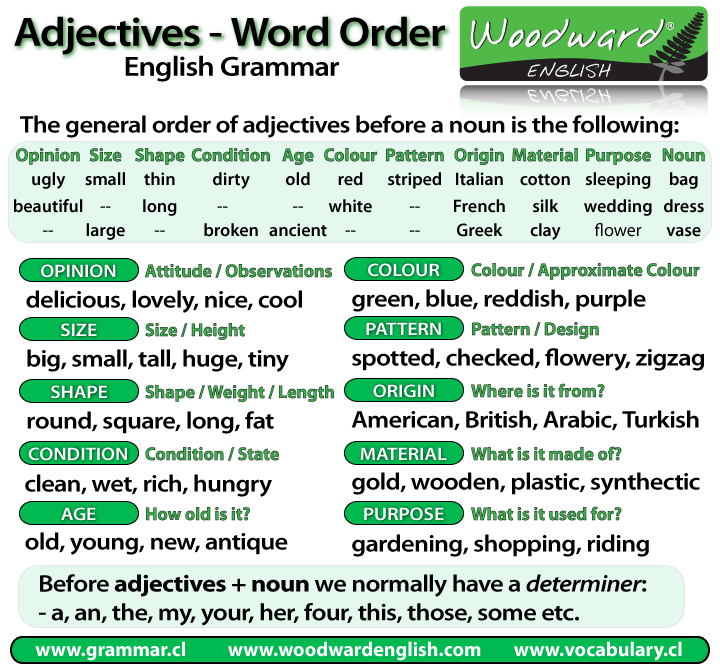

In the compound on the right, the diene is homoannular with 4 alkyl substituents. This diene group has 4 alkyl substituents (labeled 1,2,3,4) and the double bond in one ring is exocyclic to the other (adding 5 nm for an exocyclic double bond). In the compound on the left the base value is 214 nm (a heteroannular diene). These rules predict the UV absorption maximum of compounds. A diene is either homoannular with both double bonds contained in one ring or heteroannular with two double bonds distributed between two rings. One set of Woodward-Fieser rules for dienes is shown in table one. Examples are conjugated carbonyl compounds, conjugated dienes, and polyenes. The rules build the prediction on the type of chromophores present, the substituents on the chromophores, and changes due to the solvent. The rules are sometimes called the Woodward-Fieser rules, to also honor Louis Fieser. He was a Harvard University professor who won the 1965 Nobel Prize in chemistry. The rules are named after Robert Burns Woodward. They give information about the wavelength of the absorption maximum (symbol λ max ) in an ultraviolet-visible (UV) spectrum of a compound. Woodward's rules are a set of rules about how organic chemical compounds absorb ultraviolet light.


 0 kommentar(er)
0 kommentar(er)
